What is protein?
Protein word is derived from Greek word “prōteios” which means “holding first place”. This term was coined by Swedish chemist Jöns Jacob Berzelius in 1838 due to its importance in living organisms.
Protein is a highly complex macromolecule that is made up of one or more long chains of many different amino acids arranged in a specific order. It is the most abundant organic compound present in all types of cells and all parts of a cell. It takes about 50% of the total dry weight of a cell. The rich sources of protein are meat, eggs, seafood, poultry, beans, pulses, nuts, seeds, and peas.
Function and structure of protein
Functions of protein
Proteins are highly important to living organisms where they perform diverse functions. Some functions are:
1. Cell structure
Various types of proteins are involved in the building of cell structures such as cell membranes and the ribosome. Structural proteins give shape, rigidity, and stiffness to the fluid part of the cell. For example, connective tissue such as cartilage contains fibrous proteins called collagen and elastin. Similarly, hair, nails, hooves, and feathers contain keratin protein.
2. Enzymes
Enzymes that catalyze the chemical reactions and control the whole metabolism of a cell and body are protein in nature. For example, enzymes involved in the digestion of food (pepsin, trypsin, amylase, and lipase), replication of DNA (helicases), clotting of blood (thrombin), and transcription (RNA polymerase).
3. Hormones
Some hormones are made up of long chains of amino acids. Peptide hormones are involved in the regulation of metabolic processes such as maintenance of blood glucose level (insulin), growth (growth hormones), and release of bone calcium into the bloodstream (parathyroid hormone).
4. Transporters
Some proteins work as a transporter and carry different molecules (metal ions, oxygen, lipids, and carbon dioxide) from one part of the body to another part. For example, hemoglobin, a complex protein present in red blood cells, helps in the transport of oxygen from the lungs to the whole body and carbon dioxide from the whole body to the lungs.
5. Antibodies
Antibodies or immunoglobulin that defend the body against foreign substances and pathogens are also made up of protein. Some examples of antibodies include Immunoglobulin G (IgG), and Immunoglobulin A (IgA).
6. Movement
Proteins present in organs help in the movement of an organism. Muscle proteins Actin and myosin proteins present in muscles are involved in the relaxation and contraction of muscles and thus the movement of arms. Flagellin proteins present in bacterial flagella help in the locomotion of bacteria.
7. Cell cycle
Proteins are involved in the regulation and maintenance of the eukaryotic cell cycle. Replication of DNA during interphase, movement of chromosomes during anaphase, and cell division during cytokinesis are controlled by kinases and cyclins proteins.
Video lesson (Function of Protein and Structure of Amino Acid)
Structure of protein
Protein is a polymer of amino acids. Amino acid is a small organic molecule made up of carbon, nitrogen, oxygen, and hydrogen elements. There are about 170 naturally occurring amino acids, however, only 20 amino acids are involved in the synthesis of proteins.
Most bacteria and plants can synthesize all twenty amino acids by themselves, but animals including humans must get some amino acids from their diet known as essential amino acids. Essential amino acids are those that an organism cannot produce on its own.
Structure of amino acids
An amino acid consists of central carbon also known as alpha carbon to which amino group (-NH2), carboxyl group (-COOH), a hydrogen atom (-H), and R group are attached. The R group makes the side chain of amino acids and its variations make different amino acids. For example, the R group in amino acid glycine is hydrogen (-H), while in amino acid alanine it is the methyl group (-CH3) group. The smallest amino acid is glycine, while the largest amino acid is tryptophan.
During the synthesis of a protein, amino acids are linked together by the formation of a peptide bond between the H atom of an amino group and the OH group of the carboxyl group with the release of a water molecule.
Levels of protein structure
Protein structure is divided into four distinct levels.
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
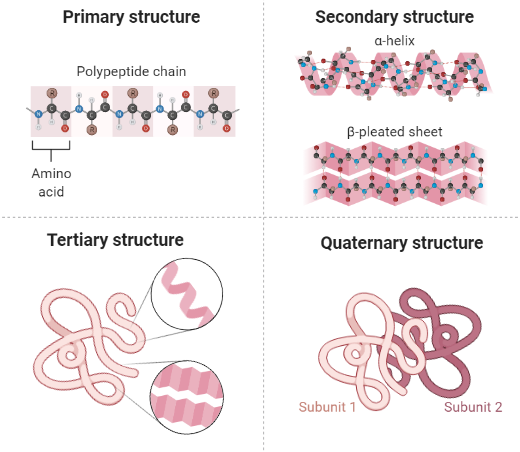
|
| Four levels of structure of protein. Created in BioRender.com |
1. Primary structure
The primary structure of protein comprises of number and sequence of amino acids in a polypeptide chain. Amino acids are held together by a peptide bond to form a stable primary structure. In the polypeptide chain, the free end containing a carboxyl group is called carboxyl terminus (C-terminus), and an amino group-containing free side is called amino terminus (N-terminus).
The primary structure of the protein is determined by a gene that is first transcribed into mRNA and then translated into a polypeptide chain.
Amino acids sequence of insulin was first studied by F. Sanger in 1943 and established the fact that protein is made up of a specific sequence of amino acids. He described that insulin is made up of 51 amino acids organized into 2 chains of 21 and 30 amino acids.
Another important protein in vertebrates is hemoglobin which is present in red blood cells and is responsible to carry oxygen. Hemoglobin is made up of 4 chains; 2 alpha chains containing 141 amino acids and 2 beta chains consisting of 146 amino acids.
The size of a protein depends on the type and numbers of amino acids that are measured in kilo Dalton (kDa). For example, the size of insulin is 36 kDa, while the size of human hemoglobin is 64.5 kDa.
The arrangement of amino acids is highly specific for each protein and is important for the proper functioning of the protein. For example, in sickle cell anemia glutamic acid (amino acid) at the 6th position of the alpha chain of hemoglobin protein is replaced with valine amino acid due to which the oxygen-carrying ability of red blood cells is halted.
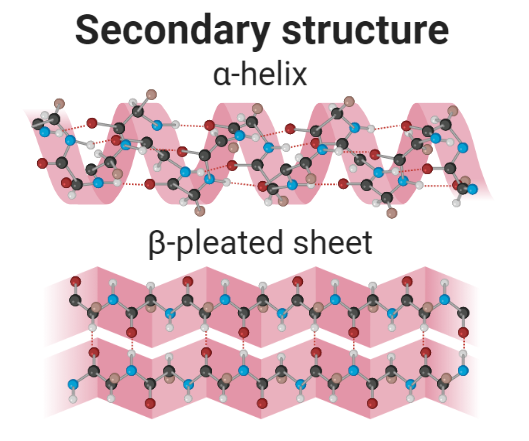
2. Secondary structure
A secondary structure is a regular structure formed due to hydrogen bonding between atoms of neighboring amino acids of a growing polypeptide. There is two main secondary structure of proteins:
- α-helix
- β-pleated sheet
1. α-helix
It is the spiral formation of amino acids of a polypeptide. The amino acids form a structure similar to the telephone line cord. In α-helix, 3.6 amino acids are organized in each turn and are held stable by hydrogen bonding. A hydrogen bond is formed between the N-H backbone and the C=O backbone of amino acid located four residues earlier along in a polypeptide chain.
2. β-pleated sheet
This secondary structure is formed due to the regular folding of a polypeptide. Hydrogen bonds are formed between N−H groups of amino acids present in one established chain with the C=O group of amino acids present in the adjacent antiparallel chains of the folded polypeptide. The β-pleated sheets have been involved in the development of the fibrils and protein aggregates detected in amyloidosis.
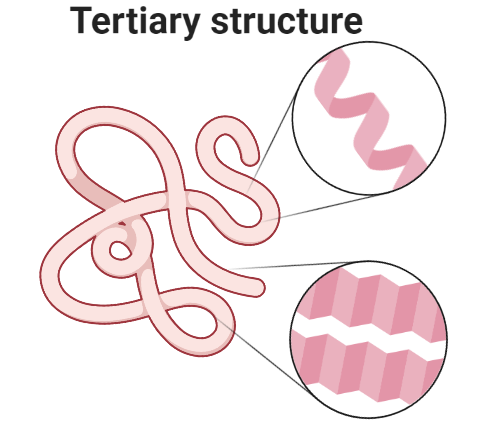
3. Tertiary structure
Tertiary structure is formed when a single polypeptide bends and folds upon itself to form a globular structure. This three-dimensional structure is maintained by three types of bonding i.e., ionic, hydrogen, and disulfide (-S-S-). In an aqueous environment, tertiary structure is the most stable conformation in which hydrophobic amino acids are buried inside, while hydrophilic amino acids are on the surface of the globular protein.
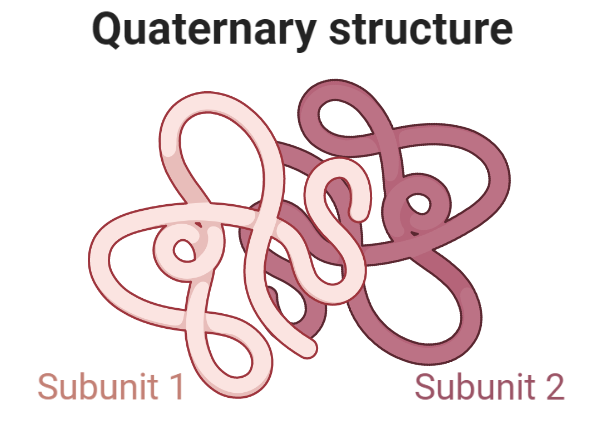
4. Quaternary structure
It is a highly complex structure of the protein. Two or more polypeptide chains are aggregated to operate as a single functional unit and called multimers. The chains in multimers are held together by hydrophobic interactions, hydrogen bonding, and ionic bonding. For example, hemoglobin is a tetramer in which four chains are aggregated together to form a quaternary structure.
Video Lesson (Struture of protein | Four Levels of Protein Structure)
Summary
Proteins are made up of amino acid chains that fold into distinctive three-dimensional structures. The structure of a protein is stabilized through bonding, and the final folded shapes of proteins are well-suited to their functions.
Some Questions and Answers
1. What are the building blocks of protein?
A. Amino acids are the building blocks of protein.
2. What are the main sources of protein?
A. Meat, fish, pulses, beans, and seafood are the main sources of protein.
3. How does protein helps in the clotting of blood?A. Enzyme thrombin which is involved in the clotting of blood is a protein in nature.
4. Who studied the amino acid sequence of insulin?
A. F. Sanger studied the amino acid structure of insulin protein for the first time in 1943 for which he received Nobel Prize.
5. Which two main structures are listed under the secondary structure of a protein?
A. The α-helix and β-pleated sheet are two main secondary structures.
6. How three-dimensional tertiary structure is maintained in protein?
A. This three-dimensional structure is maintained by three types of bonding i.e., ionic, hydrogen and disulfide (-S-S-).
7. Give two examples of protein having a quaternary structure.
A. Hemoglobin, ribosomes, and antibodies have a quaternary structure.
8. State two functions of protein in the cell.
A. They control the whole metabolism of a cell in the form of enzymes. They regulate and maintain the eukaryotic cell cycle.






0 Comments