Definition of reducing sugar
A reducing sugar is a type of carbohydrate that contains a free hemiacetal or hemiketal functional group in its molecular structure. This functional group, typically derived from an aldehyde or ketone group, can undergo oxidation-reduction reactions, leading to the donation of electrons and the potential formation of a corresponding carboxylic acid group.
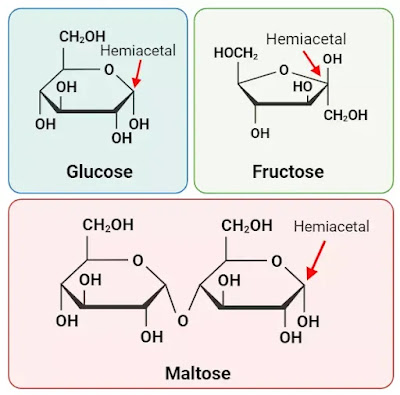
This reactivity allows reducing sugars to act as reducing agents in various chemical reactions and to yield positive results in tests that detect their presence, such as the Benedict's test or Fehling's test. Examples of reducing sugars include glucose, fructose, and maltose.
A hemiacetal group is a functional group that consists of a carbon atom bonded to an oxygen atom and a hydroxyl (-OH) group. It is formed when an aldehyde group (a carbonyl group with a hydrogen atom attached) reacts with an alcohol group (-OH) in the same molecule. Similarly, a hemiketal group is formed when a ketone group reacts with an alcohol group in the same molecule.
What does reducing sugar mean
A reducing sugar is a type of carbohydrate that has the ability to undergo a chemical reaction known as reduction. This reaction involves the conversion of the functional group within the sugar molecule, which is typically an aldehyde (or sometimes a ketone) group, into a corresponding alcohol group.
Reducing sugars are called "reducing" because they are capable of donating electrons to other molecules. In the context of a chemical reaction, reduction involves the gain of electrons. When a reducing sugar undergoes a reduction reaction, it loses the characteristic reactivity of its aldehyde or ketone group, which becomes an alcohol group.
In the context of reducing sugars, the presence of a hemiacetal group is what gives these sugars their reducing properties. This is because the hemiacetal group can undergo oxidation-reduction reactions. In particular, the aldehyde or ketone group in the hemiacetal can be oxidized, leading to the formation of a carboxylic acid group.
The term "reducing sugar" is particularly important in the context of laboratory tests and food chemistry. Some common tests, like the Benedict's test or Fehling's test, use the reducing properties of sugars to detect their presence. These tests involve the reduction of a copper ion in a reagent solution, leading to the formation of a colored precipitate. If the substance being tested contains reducing sugars, the solution will change color, indicating a positive result.
It's important to note that not all carbohydrates are reducing sugars. For instance, sucrose (table sugar) is a non-reducing sugar because it lacks a free aldehyde or ketone group and cannot participate in the reducing reaction.
Examples of reducing sugar
Here are a couple of examples of reducing sugars along with explanations of their reducing properties.
Glucose:
Glucose is a monosaccharide and a prime example of a reducing sugar. It has an aldehyde group (-CHO) at the end of its molecular structure. An aldehyde group on the carbon at position 1 reacts with a hydroxyl group on the carbon at position 5, forming a hemiacetal linkage. This is known as the glucose ring structure. The hemiacetal group in glucose can undergo oxidation reactions, making glucose a reducing sugar.
When glucose is in solution, the aldehyde group can donate electrons, making it a reducing agent. In a reducing environment, the aldehyde group can be oxidized, leading to the formation of a corresponding carboxylic acid group. This oxidation-reduction reaction is the basis for the Benedict's test and Fehling's test for reducing sugars.
Fructose:
Fructose is another monosaccharide and a reducing sugar. Similar to glucose, it has a ketone group in its structure. When it undergoes a reaction to form a ring structure, the ketone group reacts with an adjacent hydroxyl group to form a hemiketal linkage. This hemiketal group can also undergo oxidation reactions, allowing fructose to act as a reducing sugar.
Although ketones are less reactive than aldehydes, the ketone group in fructose can still undergo a redox reaction in the presence of suitable reagents. The ketone group can be converted to a secondary alcohol group, making fructose a reducing sugar. The reactivity of the ketone group in fructose is why it can participate in the Benedict's and Fehling's tests.
Maltose:
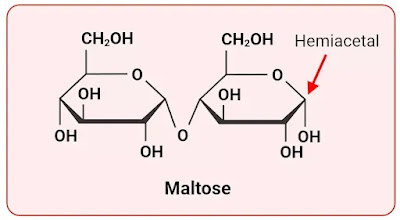
Maltose is a disaccharide composed of two glucose molecules linked together. It is a reducing sugar due to the presence of a free aldehyde group at the end of one of the glucose molecules. The other glucose molecule forms a glycosidic bond with the aldehyde group. The aldehyde group can react with oxidizing agents, demonstrating the reducing properties of maltose.
These examples demonstrate that reducing sugars contain functional groups (aldehyde or ketone) that can donate electrons, leading to oxidation-reduction reactions. This property is utilized in various biochemical and analytical tests to detect the presence of these sugars in solutions. It's important to note that non-reducing sugars, like sucrose, lack the necessary functional groups for these reactions and thus do not show the same reactivity in these tests.
Short video on what does reducing sugar mean
Tests for reducing sugars
Two common tests used to identify the presence of reducing sugars are the Benedict's test and the Fehling's test. Both of these tests take advantage of the reducing properties of certain functional groups in sugars.
Benedict's Test:
The Benedict's test is a qualitative test for the presence of reducing sugars, particularly monosaccharides and some disaccharides. Here's how it works:
Principle of Benedict's test:
Benedict's reagent, which contains copper sulfate (CuSO4) and sodium carbonate (Na2CO3) in an alkaline solution, is used as the testing reagent. The copper ions in CuSO4 can be reduced by the aldehyde or ketone functional groups in reducing sugars. When the copper ions are reduced, they form a colored precipitate of copper(I) oxide (Cu2O), which indicates a positive test result.
Procedure of Benedict's test:
Here are the steps of Benedict's test.
- Prepare the sample to be tested and mix it with Benedict's reagent.
- Heat the mixture in a water bath or directly over a flame.
- If reducing sugars are present, a color change will occur. The color can vary from blue (no reducing sugar) to green, yellow, orange, or even red (high concentration of reducing sugar).
Fehling's test:
The Fehling's test is another method to detect reducing sugars, similar to the Benedict's test. It's based on the same principle of copper ion reduction by the aldehyde or ketone groups in reducing sugars.
Principle of Fehling's test:
Fehling's reagent is made up of two separate solutions: Fehling's A (copper sulfate solution) and Fehling's B (alkaline tartrate solution). When the two solutions are mixed in equal parts, a deep blue solution is formed due to the presence of copper ions. If reducing sugars are present, they will reduce the copper ions, resulting in the formation of a brick-red precipitate of copper(I) oxide.
Procedure of Fehling's test:
Here are the steps of Benedict's test.- Mix equal volumes of Fehling's A and Fehling's B solutions to form Fehling's reagent.
- Heat the reagent solution gently to bring it to a boil.
- Add the sample to be tested and continue heating.
- If reducing sugars are present, a brick-red precipitate will form, indicating a positive result.
Both the Benedict's test and the Fehling's test rely on the reducing ability of sugars with free aldehyde or ketone groups. When these functional groups react with the copper ions in the reagents, they cause a reduction reaction and the formation of colored precipitates. These tests are useful for identifying the presence of reducing sugars in various samples, including foods and beverages.
Conclusions
Reducing sugars are carbohydrates containing free hemiacetal or hemiketal functional groups, commonly derived from aldehyde or ketone moieties. These groups can undergo oxidation-reduction reactions, acting as reducing agents.
The Benedict's and Fehling's tests exploit this reactivity. In these tests, copper ions are reduced by sugar functional groups, forming colored precipitates. A positive result indicates the presence of reducing sugars, such as glucose and fructose, providing a useful method for identifying these sugars in various substances.
Some questions and answers
1. What is a reducing sugar?
A. A reducing sugar is a type of carbohydrate that contains a free hemiacetal or hemiketal functional group in its molecular structure.
2. Which tests are used for reducing sugars?
A. Benedict's test or Fehling's test can be used to identify reducing sugars.
3. Give some examples of reducing sugar.
A. Examples of reducing sugars include glucose, fructose, and maltose.
4. Is sucrose a reducing sugar?
A. No, sucrose is not a reducing sugar because it doesn’t have hemiacetal group.





0 Comments