What is sucrose
Sucrose is a common disaccharide sugar that consists of two simple sugar molecules, glucose and fructose, chemically bonded together. It is frequently referred to as table sugar and is the sugar that is most frequently used to sweeten foods and drinks. The chemical formula of sucrose is C12H22O11.
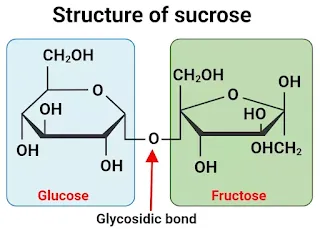
Structure of sucrose
The chemical composition of sucrose is represented by chemical formula of C12H22O11. This formula shows that it consists of 12 carbon (C) atoms, 22 hydrogen (H) atoms, and 11 oxygen (O) atoms.
At molecular level, sucrose is a disaccharide, which means it is composed of two monosaccharide molecules joined together by a glycosidic bond. In the case of sucrose, it is made up of one glucose molecule and one fructose molecule.
Glucose: Glucose is a monosaccharide with the chemical formula C6H12O6. It is a hexose sugar, meaning it has six carbon atoms. The structure of glucose can be represented as a six-membered ring with five carbon atoms and one oxygen atom.
Fructose: Fructose is also a monosaccharide with the same chemical formula as glucose (C6H12O6) but with a slightly different structural arrangement. Fructose is often represented as a five-membered ring structure.
Glycosidic Bond: The linkage between glucose and fructose in sucrose is a glycosidic bond. Specifically, it's an α,β-1,2-glycosidic bond, which means that the oxygen atom on carbon-1 of the glucose molecule is linked to the carbon-2 of the fructose molecule, and vice versa. This bond is a type of covalent bond that joins the two sugar molecules together.
In conclusion, sucrose is a disaccharide sugar made of one molecule of glucose and one molecule of fructose joined by an,-1,2-glycosidic bond. Because of this structure, sucrose has a distinctively sweet flavor that makes it a common ingredient in a variety of foods and beverages. When sucrose is taken, the digestive system's enzymes break it down into the molecules of glucose and fructose that make up the substance. These molecules are then absorbed into the circulation and utilised by the body as a source of energy.
Why sucrose is a non reducing sugar
1. No reducing ability:
Sucrose is a non-reducing sugar because it lacks the ability to reduce other substances, specifically the ability to donate electrons to another compound. This reduction capability is typically associated with sugars that contain a free aldehyde or ketone group, which can undergo a chemical reaction known as a "reduction reaction" by donating electrons.
2. No free aldehyde or ketone group
In the case of sucrose, its structure does not contain a free aldehyde or ketone group. Sucrose is composed of two monosaccharides, glucose, and fructose, which are joined together by a glycosidic linkage between the anomeric carbon atoms (carbon-1) of each sugar molecule. This glycosidic linkage results in the formation of an acetal group, and the anomeric carbon atoms are no longer free to participate in reducing reactions.
3. No reaction with redcuing agents
A carbohydrate must have a free carbonyl group that can open and undergo a reaction with another substance, such as a moderate reducing agent like Benedict's reagent or Fehling's solution, in order to be categorized as a reducing sugar. Because each of the two sugars that make up sucrose, glucose and fructose, has a free carbonyl group, they are reducing sugars:
- Glucose has an aldehyde group (a carbonyl group) at carbon-1.
- Fructose has a ketone group (a carbonyl group) in its structure.
However, when glucose and fructose are chemically bonded to form sucrose, the glycosidic linkage between them results in the formation of a new compound in which the carbonyl groups are no longer free and available for reduction reactions. Therefore, sucrose itself cannot reduce other substances and is classified as a non-reducing sugar.
Chemical assays like Benedict's test or Fehling's test can be used to determine if a sugar is decreasing or not. These tests, which look for the presence of free carbonyl groups, often yield good results for the reducing sugars glucose and fructose but negative results for the non-reducing sugar sucrose.
Video lesson on why sucrose is a non reducing sugar
Why sucrose is known as invert sugar?
Sucrose is known as "invert sugar" because it undergoes a process called inversion when exposed to acidic conditions or when acted upon by an enzyme known as sucrase. This process results in the breakdown of sucrose into its constituent monosaccharides, glucose, and fructose, which are both reducing sugars.The name "invert sugar" comes from the fact that this process causes a change in the optical rotation of the sugar solution. Sucrose itself has a positive optical rotation, meaning it rotates plane-polarized light to the right (clockwise). However, after the inversion process, the resulting mixture of glucose and fructose has a negative optical rotation, rotating the plane-polarized light to the left (counterclockwise). This change in optical rotation is where the term "invert" comes from.
Some questions and answers
1. Why is sucrose considered a non reducing sugar?
A. Sucrose is considered a non-reducing sugar because it lacks a free carbonyl group (aldehyde or ketone) that can participate in reduction reactions. The glycosidic linkage between glucose and fructose in sucrose prevents the formation of a free carbonyl group.
2. How can you test for the presence of reducing and non-reducing sugars in a solution?
A. To test for reducing sugars, you can use chemical tests like Benedict's test or Fehling's test. These tests involve heating the sugar solution with the reagent, which will cause reducing sugars to reduce the reagent, resulting in a color change. Sucrose, being a non-reducing sugar, will not yield a positive result in these tests.
3. Can sucrose be converted into a reducing sugar through a chemical reaction?
A. Yes, sucrose can be converted into reducing sugars through hydrolysis. By treating sucrose with an acid or enzyme, it can be broken down into its constituent monosaccharides, glucose, and fructose. These monosaccharides are reducing sugars due to their free carbonyl groups.
4. Why is it important to distinguish between reducing and non-reducing sugars in food chemistry?
A. In food chemistry, distinguishing between reducing and non-reducing sugars is crucial because it can reveal details about the make-up of the carbohydrates in a food sample. As reducing sugars can engage in Maillard reactions and caramelization, resulting in flavor and color changes, it can also assist predict how a food product may respond during cooking or processing.



2 Comments
I believe that sucrose structure is wrong. On the furanose on the right side, there is only a CH2OH, not that and an additional OH. Carbon 2 cyclates with carbon 5, leaving a CH2OH at carbon 6. Please correct it because it can be very confusing. Thanks in advance.
ReplyDeleteI SAID THE SAME THING LMAO THIS DUDE. so dangerous for students i cant believe it
Delete