Define osmosis
Osmosis is a passive biological process involving the movement of water molecules across a selectively permeable membrane from an area of higher water potential to an area of lower water potential.
The goal of osmosis is to equalize the concentration of water on both sides of the membrane, maintaining cellular and organismal balance.
It is a crucial mechanism in biological systems, influencing cell volume, nutrient uptake, and overall cellular homeostasis.
Effect of osmosis on plant cells
Osmosis plays a crucial role in the behavior and health of plant cells. The effects of osmosis on plant cells are primarily observed in different types of solutions.
 |
| Image created in BioRender.com |
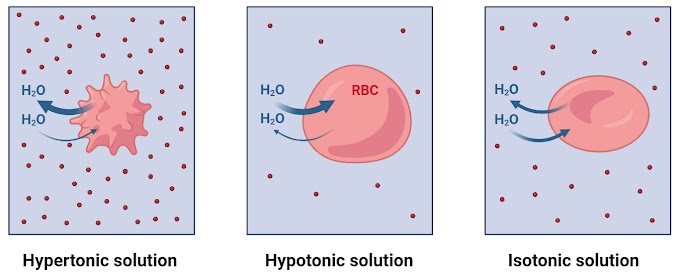
Isotonic Solution
In an isotonic solution, where the concentration of solutes is the same inside and outside the plant cell, there is no net movement of water. The cell maintains its normal shape and turgor pressure. Isotonic conditions are ideal for plant cells, allowing them to function optimally.
Hypotonic Solution
In a hypotonic solution, where the concentration of solutes is lower outside the cell than inside, water moves into the cell through osmosis. This influx of water increases the turgor pressure within the cell, causing it to swell and become firm.
Hypotonic solutions are generally beneficial for plant cells, contributing to turgidity and maintaining cell rigidity. This condition is important for supporting the plant structure, preventing wilting.
Hypertonic Solution
In a hypertonic solution, where the concentration of solutes is higher outside the cell than inside, water moves out of the cell. This loss of water leads to the shrinking of the cell and, in extreme cases, plasmolysis, where the cell membrane pulls away from the cell wall.
Hypertonic conditions can be detrimental to plant cells, causing wilting and negatively impacting cell function.
Summary
In summary, osmosis influences the water balance and turgor pressure within plant cells. Proper osmotic conditions, such as those found in isotonic or slightly hypotonic environments, contribute to cell health and turgidity. However, exposure to hypertonic solutions can lead to water loss, cell shrinkage, and potential damage to the plant. Maintaining an appropriate balance of water through osmosis is essential for the overall health and function of plant cells.Explanation of effect of osmosis on plant cells using example
Let's use an example to illustrate the effects of osmosis on plant cells:
Imagine you place a plant cell, such as a cell from an onion bulb, into a container of distilled water, which is a hypotonic solution compared to the cell's internal environment. In this case the concentration of solutes in the distilled water is lower than the concentration inside the plant cell. Water molecules move into the plant cell through the cell membrane, driven by the concentration gradient. This is an example of osmosis.
The influx of water causes the plant cell to swell and become turgid. The cell membrane presses against the cell wall, and the cell becomes firm.
The increased turgor pressure within the cell, resulting from water uptake, helps maintain the shape and rigidity of the cell. Turgor pressure is crucial for supporting plant structures, such as leaves and stems. This scenario is representative of a healthy state for plant cells, as the turgid condition prevents wilting.
In natural environments, plant cells are often exposed to soil solutions that can be hypotonic, facilitating the uptake of water through osmosis and contributing to the overall health and structure of the plant.
Some questions and answers
1. What happens to a plant cell placed in an isotonic solution?
A: In an isotonic solution, there is no net movement of water, and the plant cell maintains its normal shape and turgor pressure.
2. Describe the effects of osmosis on a plant cell in a hypotonic solution.
A: In a hypotonic solution, water moves into the plant cell, causing it to swell, become turgid, and maintain a firm shape.3. How does a hypertonic solution affect a plant cell through osmosis?
A: In a hypertonic solution, water moves out of the plant cell, leading to cell shrinkage and, in extreme cases, plasmolysis.4. Why is turgor pressure important for plant cells?
A: Turgor pressure, resulting from water uptake through osmosis, helps maintain the shape, rigidity, and structural integrity of plant cells.
5. How does osmosis contribute to preventing wilting in plant cells?
A: Osmosis in hypotonic solutions leads to the uptake of water, increasing turgor pressure, and preventing wilting by maintaining cell turgidity.
6. Explain the concept of plasmolysis in the context of hypertonic solutions and plant cells.
A: Plasmolysis occurs when a plant cell loses water in a hypertonic solution, causing the cell membrane to detach from the cell wall.7. What is the significance of osmotic conditions for the overall health of plant cells?
A: Proper osmotic conditions, such as those in isotonic or slightly hypotonic environments, are essential for the health and function of plant cells, contributing to turgor pressure and structural stability.
8. How might changes in osmotic conditions affect plant cells in different environments?
A: Changes in osmotic conditions, such as exposure to hypertonic soil solutions, can lead to water loss, cell shrinkage, and potential damage to plant cells, impacting their overall health.



0 Comments